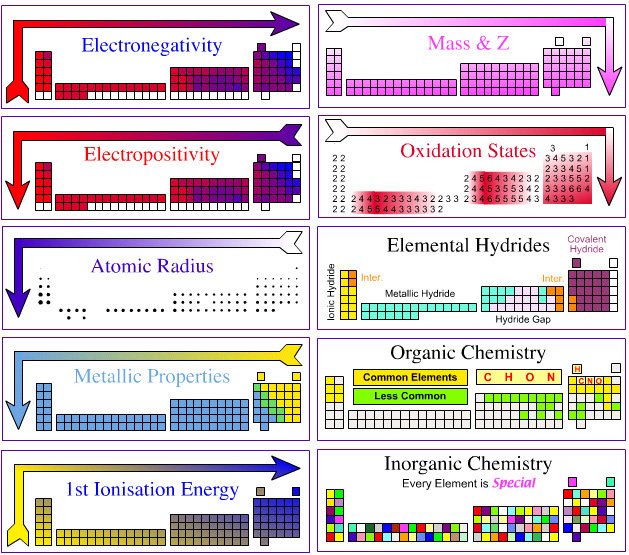
Today in class, we learned about the Periodic Trends. The trends tells us the ralationship between elements on the table. For example there are eight important trends we need to now. There is the Metallic Properites trend, Atomic Radius trend, Ionization energy trend, electronegativity trend, reactivity trend, ion charge trend, melting/boiling point trend and the density trend.
Metallic properties trend
-elements tend to be more metallic in nature toward the left side of the periodic table
-metallic properties like malleability are result of the little valance electrons
-usally low ionization energies
-elements more metallic to the left side of the stair case
-elements more metallic when down a group
Atomic Radius Trend
-radius decreases when reading down from a group
-radius increases when reading from left to right of a row
-elements have higher enegy level at the bottom. The atom is more sprend apart making the radius bigger
-elements atomic radius decreases from left to right of the row because the atomic number increases making the nucleaus more attractive to the other electrons. Thus the atom is more packed together and not wide apart

Ionization of energy trend
-is the process of removing one or more elctrons from an atom to produce an ion
-opposite trend of atomic radius
-energy becomes bigger from left to right of the row because the atom is more tightly packed since the electrons are attracted to the positve nucleuas with protons. Thus harder to remove an electron from the atom
-energy decreases as reading down the group because electrons more far apart because of higher energy level. Thus electrons are easier to be remove and requir less energy

Electronegativity trend
-is the ability to attract electons from a neighbouring atom
-same trend as ionization energy
-elements to the right of the row has high ionization energy. Thus they have higher attraction to their neighbors
-elements down a group has lower ionization energy since their atom is not tightly packed together. Thus they are loose and will not have enough energy to attract their neighbors

Reactivity Trend
-is how readily an element will react with other elements
-the reacticity trend for metals is different from non metals. For example when you read down the alkaline metal group the ionization energy decreases. Therefore it is easier for the element to loos its valace electron and that will make the element more reactive
-for non metals, they tend to want to again electrons. As you read upward the halogen group the element becomes more reactive since its got higher ionization level. This means that elements have a lower tendency to lose electrons and a greated tendency to gain them. Thus elemts become more reactive as you proceed up the group for non metals

Ion Charge
-Elements ion charges depend on thier group

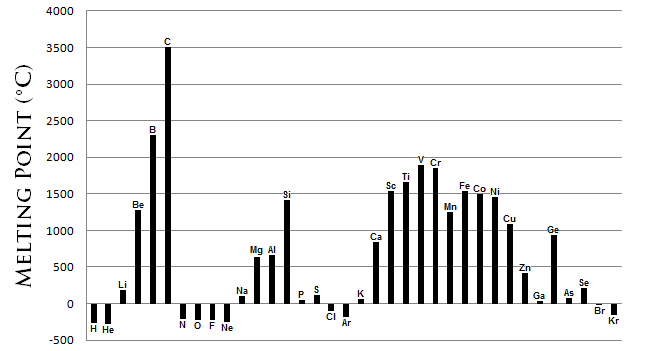
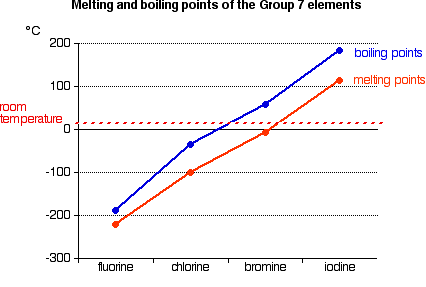
Melting point/boiling point trend
-is the temperature at which it changes from solid to a liquid
-melting point of a substance depends the stregh of their bond. If the bond is weak the melting point iwll be lower
-melting point for metals usually decreases as read down a group
-melting point for metals usually increases when moving to the right of the row until it reach the middle
-for the halogen group and the noble gas group, the melting point increases as read down the group
-element at the center of the circle has the highest melting point
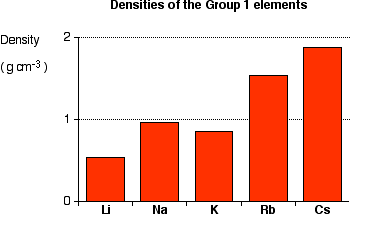
Density
-The density usually increase by the atomic number